Dan Schroeder, Physics Department, Weber State University
The following is a slightly-edited transcript of a lecture that I gave at a meeting of physics educators in June 2000.
Good morning. Before beginning this talk, I'd like to get a couple of preliminary matters out of the way. One is my credentials. Why was I invited to give a talk on the subject of thermal physics? Although it may seem strange, I have absolutely no professional expertise in thermal physics. Thermal physics was my weakest subject, among theory courses, in both college and graduate school. I failed the statistical mechanics section of my PhD qualifying exam. I've never used any thermal physics in my research--I'm an elementary particle physicist. And the first time I taught thermal physics, eight years ago, it was a total disaster, at least from the students' perspective.
I hope, though, that I learned a few things while teaching that course, and that I've continued to learn some things about teaching thermal physics in the eight years since then. I did manage to convince Addison-Wesley that I knew enough about the subject to write a textbook on it, and I'm sure it was because of my book that I was invited to talk to you today.
But when I was first asked to speak at this conference, my immediate thought was, "Everything I know about thermal physics is already in the book--if I knew anything else, I would have put it into the book!" And so I was faced with a decision. I could either try to teach you some of the physics I've learned, which would mean standing up here and reading you a section out of the book, or I could talk about something else besides physics. I'm sorry to report that I've decided on the latter. I'm not going to teach you any physics. Instead, I'm going to give one of those fuzzy talks about how we teach physics, and how we teach thermal physics in particular. I'm also going to express some opinions about how we should teach thermal physics. I hope you'll take these opinions with a grain of salt, realizing that they come from an amateur and an outsider to the field in which so many of you are professional experts.
The title of my talk is, "The undergraduate thermal physics course: Who should take it and why?" To make a long story short, my answers to these two questions will be as follows: Everyone who possibly can take a thermal physics course should take it--not just physics majors, but also other students of science and engineering and everything else (provided they have the prerequisites). Why? For lots of specific, practical reasons, but fundamentally because thermal physics tells us a great deal about how the world works, and everyone who lives in this world should know something about how it works.
Now in order to explain my answers to these questions more fully, I'm going to need to discuss some other commonly-heard answers and what's wrong with them. All too often, the answers we hear to these questions are as follows: Who should take thermal physics? Physics majors should take thermal physics. Why? Because they'll need to know thermal physics when they get to graduate school. Do these answers sound silly to you? I hope so. But just in case they don't, let me spell out in some detail just why these are such bad answers.

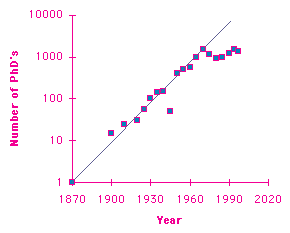
Here's a graph that you've seen before if you attended David Goodstein's Oersted Medal acceptance speech at the 1999 AAPT Winter Meeting in Anaheim, or if you read his speech in the March 1999 issue of the American Journal of Physics. The graph shows the number of physics PhD's awarded in the U.S. each year, as a function of time. (The data is from Goodstein's paper and the AIP web site.) Notice the logarithmic scale. The first PhD was awarded in about 1870, and from then on the number grew exponentially for an entire century, doubling every decade until it finally topped out at a little more than a thousand PhD's per year. During the era of exponential growth, in order to sustain the exponential growth, it was the duty and obligation of each academic physicist to produce approximately ten more academic physicists (on average), and needless to say, doing so took up nearly all of the time and energy of academic physicists.
But, of course, the era of exponential growth couldn't last forever. As Goodstein pointed out, the specific reasons why the exponential growth ended around 1970 are totally irrelevant: Exponential growth is fundamentally unsustainable, and has to end sooner or later. (That's also known as Bartlett's first law.) Those among us who yearn for a return of the era of exponential growth, when our primary duty as academic physicists was to produce more academic physicists, are simply kidding themselves. Whether we like it or not, we live in the era of stability and sustainability, when each of us, on average, has to turn out only one replacement, only one future academic physicist. The rest of our time and energy will have to be spent doing something else.
The obvious candidate for this "something else" is teaching our undergraduate students. But as the next graph shows, the number of undergraduate physics degrees awarded in the U.S. hasn't increased since 1970 either--in fact it has declined to pre-1960 levels. (This graph comes from the AIP web site.) But even if the number of undergraduates stops declining and remains at around 4000 per year during the coming decades, we as physics teachers should be concerned. Let me try to convince you of this by comparing the number 4000 to two other numbers.
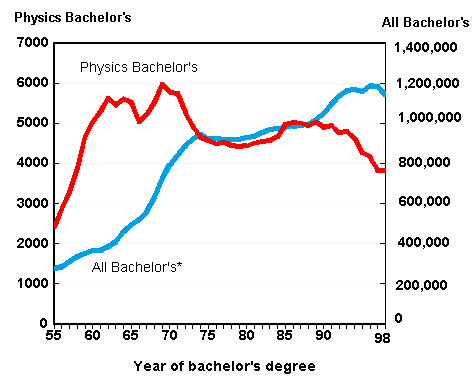
The first number to use as a comparison is the number of full-time physics faculty members at schools in the U.S. that grant bachelor's degrees in physics. That number is currently about 8300. In other words, 4000 graduating physics majors per year is less than one half of a student per faculty member. Sooner or later, departments with such embarassingly low student/faculty ratios are going to be targeted by adminstrators for cost-saving cutbacks. The physics major programs at many colleges and universities may be eliminated completely. That means that some of us will be losing our jobs, and, more importantly, students at many schools will no longer even have the opportunity to major in physics.
The second number we can use for comparison is the total number of bachelor's degrees, in all fields, awarded in the U.S. each year. That number is about 1.2 million. 4000 is smaller than 1.2 million by a factor of 300, so only 1/3 of 1% of all bachelor's degrees awarded in the U.S. are in physics. For every student who graduates with a degree in physics, there are 300--that's right, 300--who are missing out, who are graduating from college yet are ignorant of the many wonders taught in our upper-division physics courses, including thermal physics. (By the way, I don't have any statistics on how many non-majors are currently taking courses in thermal physics, but I think it's safe to assume that the number is fairly small.)
What I'm proposing is that the number of students majoring in physics each year in the U.S. could and should be much higher than 4000, and furthermore, that many non-physics majors should also be taking upper-division physics courses, especially thermal physics. Surely it isn't too much to hope that we might entice 1% of all college graduates to take a course in thermal physics. Yet 1% of all college graduates would be 12,000 students per year, roughly three times as many as are currently taking such a course. (Just think of what that would do for the viability of our departments, not to mention for the royalty checks of thermal physics textbook authors!)
Supposing that we can entice several thousand more students to take thermal physics each year, what good will it do them? The vast majority of these students will not grow up to become academic physicists, or research physicists of any sort. Many of them will become chemists or biologists or earth scientists or engineers. Some will become doctors or lawyers or managers or politicians or technicians or teachers or homemakers. Most of them will find that an understanding of thermal physics makes them better able to do their daily work. All of them will find that an understanding of thermal physics illuminates their daily lives and helps them make sense of the world they live in.
Thus I've come back to the title of my talk. Who should take thermal physics? Everyone who possibly can! Why? For countless specific and practical reasons (like needing to know about efficiency of engines or the greenhouse effect or Fermi gases), but more generally because thermal physics tells us an enormous amount about how the world works, and everyone benefits, both materially and spiritually, from understanding how the world works.
In a moment, I'll give some suggestions on how we can encourage more students to take thermal physics. But first, let me address three possible objections that may be occurring to some of you.
First, you may be thinking that tens of thousands of students each year are already learning the basics of thermal physics in our introductory physics courses. Shouldn't this be enough thermal physics for the average citizen? After all, all the average citizen needs to know about thermal physics is something about energy conservation and perhaps something about limits on efficiencies of engines and such. In a good introductory physics course, like the ones taught by Tom Moore and Ruth Chabay and Bruce Sherwood, students will learn these things. But even in the best of these courses, the amount of time spent on thermal physics won't be more than four or five weeks, and, in my experience, it's a rare student who feels truly comfortable with any subject after studying it only four or five weeks. Proficiency in thermal physics, just like proficiency in playing piano, requires a great deal of practice. Five weeks of piano lessons is long enough for a student to practice a few scales and learn a few pieces out of a beginner's lesson book, but not long enough to learn to play Chopin etudes or Scott Joplin rags. Similarly, five weeks of thermal physics may be enough to give students some appreciation of entropy, but it is not long enough for them to learn how to calculate the entropy of more than one or two toy systems. It is not long enough for them to become proficient at manipulating very large numbers, or at applying Boltzmann factors. And it is not long enough for them to see enough different applications of the subject to appreciate its breadth and applicability to most of the world around them. And yet, society needs citizens who can apply the principles of thermal physics with confidence. We need more dieticians who truly believe in energy conservation. We need more politicians who understand the limits of technology. We need more teachers who can explain why evolution does not violate the second law of thermodynamics. An introductory physics course may be better than nothing, but let's hope that at least some of these professionals will also take upper-division courses in thermal physics.
This brings me to the second possible objection to my proposal. Given that more than 4000 students per year should learn more thermal physics than we teach in our introductory courses, you may be wondering why the non-physics majors can't just learn their thermodynamics in a course in some other department, like physical chemistry or engineering thermodynamics. And it's true: They can learn a great deal in these courses. My claim is that for many of them, such an applied course is not going to be enough. They should also take a thermal physics course from the physics department. Why? Because in practice, these "applied" courses tend to focus on skills for solving a rather narrow range of problems, not on the robust understanding that you need to apply the subject in new, unfamiliar situations. A few years ago I sat in on the physical chemistry course at my own university, which was unquestionably useful in teaching students how to manipulate H's and G's, but moved through the long list of topics much too quickly to leave students with much understanding of how it all fit together. Recently I've also spent quite a bit of time looking through textbooks on thermodynamics for engineers, chemists, and earth scientists. Many of these books are outstanding in their own ways, but they are simply not intended to impart a physicist's understanding of entropy or even energy. I've been forced to conclude that thermal physics is too important to be left entirely to the teachers of engineering, chemistry, and earth science. In my own thermal physics classes I've had quite a few students who were majoring in these subjects, and practically every one of these students has personally thanked me for finally teaching them what entropy is.
A third possible objection to my proposal might be that to make our thermal physics courses interesting and accessible to non-physics majors, we would have to water them down and omit certain topics that are essential to our PhD-bound physics majors. This claim may sound plausible, but I strongly disagree. At least in a first course in thermal physics, lasting a semester or perhaps two quarters, these two groups of students will be best served by exactly the same collection of topics: the laws of thermodynamics, the statistical interpretation of entropy, engines, refrigerators, chemical reactions, phase transformations, the Boltzmann distribution, quantum gases, and various applications. You would be hard-pressed to find a thermal physics question on the GRE that isn't covered by this list of topics. Furthermore, there's plenty of material on this list to completely fill a whole semester and more without going beyond a sophomore- to junior-level treatment that should be equally accessible to majors and nonmajors alike.
The remaining question is "How?". How can we entice more students to take courses in thermal physics? Some of the barriers to their doing so are institutional, and I won't have much to say about these. For instance, I know of at least one university where students who have taken engineering thermodynamics are not even allowed to receive credit for taking thermal physics! Obviously we should lobby for the removal of such institutional barriers. Another barrier to many students is our introductory physics courses, which too often leave students with the false impression that physics is too hard, or at least too hard to really understand. I won't say any more about this problem either, although it is a very serious one that we absolutely must address. What I do want to discuss in some detail is how we should change the thermal physics course itself, to make it attractive to a wider range of students. Each of us needs to look at our own course, and ask what elements of the course encourage students to take it, or discourage them from taking it. We need to view our students not as a captive audience, taking the course to satisfy a requirement, but rather as intelligent shoppers looking for a course that will best meet their needs. Therefore, we need to pay careful attention to many details of our courses, including the level, the prerequisites, the clarity of presentation, the amount of abstraction, and, most importantly, the specific applications that are covered.
The level of the course, as I've already implied, should be such that students who have taken no physics beyond an introductory year, and no math beyond multivariable calculus, can take the course and succeed in it. I don't think these requirements constrain us much at all. We should, however, be aware of the fact that while our students may have seen multiple integrals in spherical coordinates before, most of them are not yet comfortable setting up such integrals from scratch. And we should watch our language to avoid unnecessary jargon, such as the phrase "stationary quantum states", which appears on the very first page of the thermal physics textbook that I used when I was an undergraduate.
Regarding clarity of presentation, I don't think there are any magic prescriptions, but please remember that concepts such as entropy and enthalpy and free energy and chemical potential can be explained in clear, vivid English. Entropy is the logarithm of the number of ways of arranging things. Enthalpy is the energy of the system plus the work you need to do to make room for it. Free energy is the work you can extract by annihilating the system, not including any heat that you must dump into the environment to get rid of the system's entropy. Chemical potential is a measure of the tendency of a system to give up particles, just as temperature measures its tendency to give up energy and pressure measures its tendency to expand. Even the partition function, that most abstract of thermodynamic quantities, is more or less just the number of states accessible to a system when it's held at constant temperature. None of these verbal interpretations are particularly hard to remember, and yet, all too often, they tend to get buried in the mathematics.
Too much abstraction can be a problem for our students, not just when we introduce new concepts, but also when we derive important results. We should introduce a vivid application along with every new topic: Diesel engines with adiabatic compression; batteries with Gibbs free energy; high-altitude cooking with the Clausius-Clapeyron equation; stellar spectra with Boltzmann factors; tungsten filaments with blackbody radiation. Any topic for which we can't find an immediate and relevant application should be cut from the course. There's too much material to cover in one semester anyway, and we should never try to justify piece of formalism with the lame excuse, "you'll need to know this when you get to graduate school."
Finally, let me say more about applications. Thermal physics is so rich in applications that we can never cover all of them in a first course, but I strongly feel that we need to cover as many as we can. Furthermore, we need to be careful to include applications that will interest all of our students, not just a few of them. In the following table I've tried to divide some of the common applications of thermal physics into two categories: esoteric applications, and real-world applications.
|
Esoteric Applications
|
Real-World Applications
|
| paramagnetism | cooking |
| liquid helium | weather |
| Bose-Einstein condensation | glucose metabolism |
| critical exponents | automobile engines |
| black holes | household refrigerators |
| early universe | solar radiation |
| incandescent lights | |
| lasers | |
| antifreeze | |
| acid rain | |
| semiconductors |
Every one of the esoteric applications is absolutely fascinating: paramagnetism with its mathematical elegance and counterintuitive negative temperatures; liquid helium with its intricate phase behavior and modes of excitation; Bose-Einstein condensation and the ingenious cooling techniques used to achieve it; critical exponents and their calculation through renormalization group techniques; and various astrophysical applications such as black holes and early universe cosmology. By all means, cover as many of these applications as you have time for in your undergraduate thermal physics courses. But remember that every one of these applications is extremely esoteric: Not one of them has any direct relevance to our daily lives. Many of these phenomena happen only at the sub-kelvin temperatures found nowhere in the universe outside of physics laboratories. Critical exponents are notoriously difficult to measure, even in the most careful experiments. The nearest black hole is probably hundreds of light-years away, and the early universe was, let's face it, more than ten billion years ago. No matter how fascinating these applications are in and of themselves, the fact is that many students want to study subjects that are not only fascinating but also relevant.
So please, in your thermal physics courses, be sure to also cover plenty of relevant, real-world applications. Study how thunderclouds form by adiabatic cooling of rising air, with cloud droplets nucleating around dust particles when the temperature drops to the dew point. Estimate what the temperature of earth's surface would be were it not for the greenhouse effect, and explore the implications of adding more greenhouse gases to our atmosphere. Analyze the human body as a thermodynamic engine, or rather a fuel cell, extracting a portion of the free energy released as sugars are metabolized into carbon dioxide and water. Explain how an ordinary household refrigerator works, before you launch into a discussion of helium dilution refrigerators and adiabatic demagnetization. And calculate the number of conduction electrons in a semiconductor as a function of temperature, to understand how the behavior of semiconductors can be so different from that of ordinary metals.
In conclusion, I'd like to emphasize that physics is about understanding the whole world, including the world of our everyday lives, and also including those parts of the world that have been spun off into disciplines of their own such as biology and meteorology and electronics. Rather than building barriers between physics and these allied disciplines, I'm suggesting that we should make as many connections to them as we can. If we succeed, we'll not only ensure the long-term health of our departments, but also give many more students the truly useful education in thermal physics that they need and deserve.
Posted July 27, 2000.