





 |
 |
 |
 |
 |
 |
 |
 |
 |
| Q1: temp photosphere | Q2: spots | Q3: corona | Q4: limb | Q5: next solarmax | Q6: climate | Q7: neutrinos | Q8: Homestake | Q9: Homestake |
| Lecture slides |
 This is the photosphere, the Sun you usually see (except that you should NEVER EVER look directly at the Sun!).
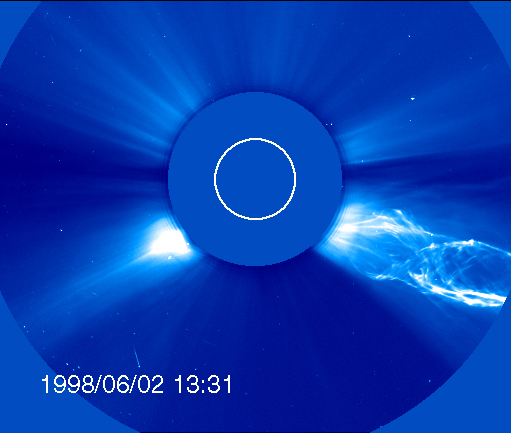
When we have a solar eclipse, we can see the corona:
This is the photosphere, the Sun you usually see (except that you should NEVER EVER look directly at the Sun!).
When we have a solar eclipse, we can see the corona:
 From the picture of the photosphere, we can figure out a LOT about the Sun. But first, we need a quick digression.
You can find the temperature of an object from the color. Higher
energy light = bluer = higher temperature. You know this already, from
working with bunsen burners in high school chemistry class.
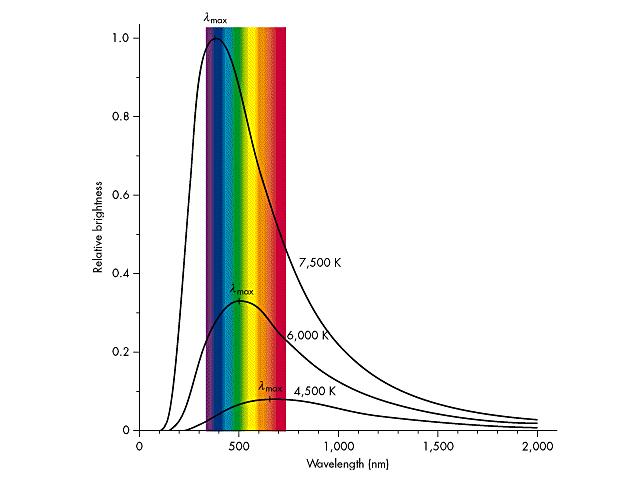
Here is the blackbody curve, which shows this relationship. The
amount of energy released by the object in a given color is plotted vs.
color on the x-axis. This is depicted for several different
temperatures:
From the picture of the photosphere, we can figure out a LOT about the Sun. But first, we need a quick digression.
You can find the temperature of an object from the color. Higher
energy light = bluer = higher temperature. You know this already, from
working with bunsen burners in high school chemistry class.
Here is the blackbody curve, which shows this relationship. The
amount of energy released by the object in a given color is plotted vs.
color on the x-axis. This is depicted for several different
temperatures:
 So, looking at the two pictures of the Sun, we find IMMEDIATELY,
that the temperature of the surface of the Sun is 5800 K. The corona is
hotter. Sunspots are cooler. AND, the temperature of the photosphere
increases as you move towards the center. Does this seem weird? Yes.
Because the temperature of the Sun cools down as the radius increases,
and then suddenly increases again! Why? We don't really know. Could be
lots of different things, but no one has yet been able to make a good
description that holds up under scrutiny.
Let's take a closer look.
So, looking at the two pictures of the Sun, we find IMMEDIATELY,
that the temperature of the surface of the Sun is 5800 K. The corona is
hotter. Sunspots are cooler. AND, the temperature of the photosphere
increases as you move towards the center. Does this seem weird? Yes.
Because the temperature of the Sun cools down as the radius increases,
and then suddenly increases again! Why? We don't really know. Could be
lots of different things, but no one has yet been able to make a good
description that holds up under scrutiny.
Let's take a closer look.
 This may or may not be connected to the Earth's climate.
This may or may not be connected to the Earth's climate.


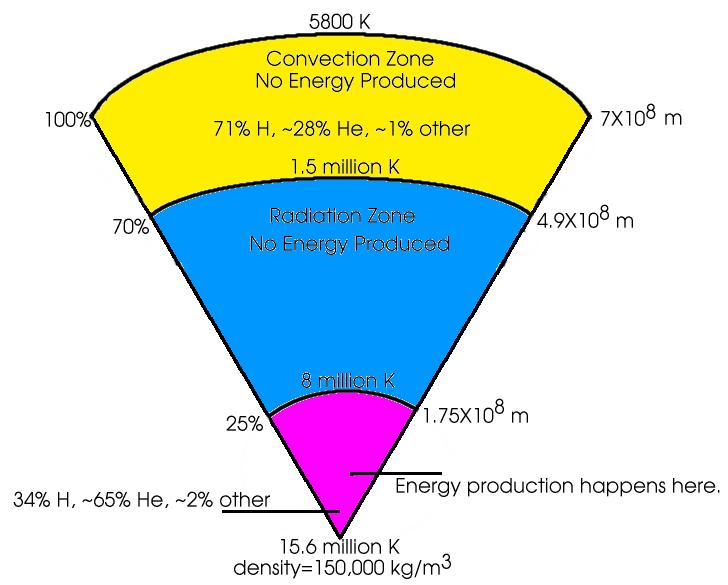
 In the deep core, the temperature is extremely high (measured
here in Kelvins, a particular temperature scale, like Celsius or
Fahrenheit. Once you are talking about temperatures this high though,
it doesn't really matter which scale you are using. The interior of the
Sun is HOT!). The pressure is also very high, compressing the mass to a
density of 150,000 kg/m3.
This is 150 times the density of water, and about 30 times the density
of rock. You would never be able to move through material this dense.
As you move out through the Sun, the temperature and the density fall
smoothly. Once the temperature drops below 8 million K, the energy
produced can travel more easily out through the star by radiative
diffusion.
The energy that we see leaving the Sun has traveled a tortuous path to
get out. Here we broke for a few minutes to have a demonstration of radiative diffusion.
The styrofoam ball did not travel from the interior of the Sun to the
exterior in any sort of sensible way, and it took a very long time. In
the Sun, the problem is extreme because of the high densities there.
Each photon (bit of light) that wants to come out of the Sun travels
only about 10-6 m before being absorbed by an atom, which
'spits it out' in a random direction. This means that it can take about
170,000 years for an individual photon to find its way out of the Sun.
Some are faster, and some are slower, but on average, this is how long
it takes. The point is that the energy you see now leaving
the Sun actually was produced from mass 170,000 years ago, before man
even existed. So we can't look at the surface of the Sun and know
what's happening inside NOW. At least not by looking at the light.
Once the temperature falls to 1.5 million K, convection can develop. This is the boiling pot method of transferring energy.
How do we know this? Obviously, I didn't go there, and take a
sample... We have figured this out from a few key pieces of evidence,
plus a lot of thinking hard about what makes energy. First, we know
that the Sun has been shining for billions of years. How do we know
this? Because the fossil record shows that there's been life on Earth
for at least 3.5 billion years. Presumably, that life could not have
existed without the Sun, about as bright as it is now. Only one known
source of energy can make as much energy as the Sun gives off for as
long as it's been shining: NUCLEAR FUSION.
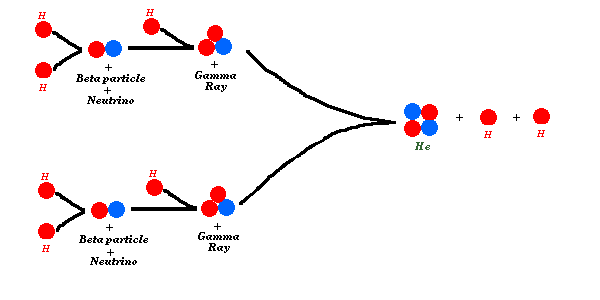
Nuclear fusion is the process of taking light atoms, and smashing them
together to make heavier ones. In particular, stars like the Sun shine
by fusing hydrogen into helium. The primary method of doing this is by
the proton-proton chain:
In the deep core, the temperature is extremely high (measured
here in Kelvins, a particular temperature scale, like Celsius or
Fahrenheit. Once you are talking about temperatures this high though,
it doesn't really matter which scale you are using. The interior of the
Sun is HOT!). The pressure is also very high, compressing the mass to a
density of 150,000 kg/m3.
This is 150 times the density of water, and about 30 times the density
of rock. You would never be able to move through material this dense.
As you move out through the Sun, the temperature and the density fall
smoothly. Once the temperature drops below 8 million K, the energy
produced can travel more easily out through the star by radiative
diffusion.
The energy that we see leaving the Sun has traveled a tortuous path to
get out. Here we broke for a few minutes to have a demonstration of radiative diffusion.
The styrofoam ball did not travel from the interior of the Sun to the
exterior in any sort of sensible way, and it took a very long time. In
the Sun, the problem is extreme because of the high densities there.
Each photon (bit of light) that wants to come out of the Sun travels
only about 10-6 m before being absorbed by an atom, which
'spits it out' in a random direction. This means that it can take about
170,000 years for an individual photon to find its way out of the Sun.
Some are faster, and some are slower, but on average, this is how long
it takes. The point is that the energy you see now leaving
the Sun actually was produced from mass 170,000 years ago, before man
even existed. So we can't look at the surface of the Sun and know
what's happening inside NOW. At least not by looking at the light.
Once the temperature falls to 1.5 million K, convection can develop. This is the boiling pot method of transferring energy.
How do we know this? Obviously, I didn't go there, and take a
sample... We have figured this out from a few key pieces of evidence,
plus a lot of thinking hard about what makes energy. First, we know
that the Sun has been shining for billions of years. How do we know
this? Because the fossil record shows that there's been life on Earth
for at least 3.5 billion years. Presumably, that life could not have
existed without the Sun, about as bright as it is now. Only one known
source of energy can make as much energy as the Sun gives off for as
long as it's been shining: NUCLEAR FUSION.
Nuclear fusion is the process of taking light atoms, and smashing them
together to make heavier ones. In particular, stars like the Sun shine
by fusing hydrogen into helium. The primary method of doing this is by
the proton-proton chain:
 Or, an animated version:
Or, an animated version:
 The Beta particle in these images is sometimes written
The Beta particle in these images is sometimes written 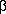 +, and is more properly called a positron.
A positron is an electron with a positive charge, and belongs to a
class of particles called 'anti-matter'. Anti-matter. No kidding. As
you know from Star Trek, when matter meets anti-matter, both particles
are destroyed, and light comes out. So inside of the Sun, positrons are
being created, which then run into electrons, and both are destroyed,
producing a gamma ray (see below).
The neutrino,
+, and is more properly called a positron.
A positron is an electron with a positive charge, and belongs to a
class of particles called 'anti-matter'. Anti-matter. No kidding. As
you know from Star Trek, when matter meets anti-matter, both particles
are destroyed, and light comes out. So inside of the Sun, positrons are
being created, which then run into electrons, and both are destroyed,
producing a gamma ray (see below).
The neutrino,  , is a
teeny-tiny particle, with very little mass, that travels very close to
the speed of light. It has no charge, and barely interacts with
ordinary matter. A typical
, is a
teeny-tiny particle, with very little mass, that travels very close to
the speed of light. It has no charge, and barely interacts with
ordinary matter. A typical  can travel through 3 light years of lead as though it wasn't even there. There are 1016
(10,000,000,000,000,000) neutrinos passing through your body every
second, and you don't even know it. They go in one side and out the
other, and don't even slow down.
A Gamma Ray is really just a high energy bit of light, like an X-ray, only with even more energy.
So you can see that a lot of energy comes out of this reaction.
There's the energy to make the neutrino, and send it flying away, and
the energy in the two gamma rays (one resulting from the
positron-electron annihilation). Where did this energy come from?
The helium atom which we end up with is actually less
massive than the four hydrogen atoms that we began with! All this talk
about mass and energy should make you think immediately (ok, maybe not immediately!) of the most famous formula ever:
E=mc2
In this equation, E stands for energy, m stands for mass, and c is the speed of light, 3X108
m/s. What it says is that mass is just another form of energy, and that
you can turn energy into mass, and mass into energy, according to the
formula. This was one of Einstein's greatest contributions to humanity,
figuring out that mass and energy are really just different forms of
the same thing.
A Gamma Ray is really just a high energy bit of light, like an X-ray, only with even more energy.
So you can see that a lot of energy comes out of this reaction.
There's the energy to make the neutrino, and send it flying away, and
the energy in the two gamma rays (one resulting from the
positron-electron annihilation). Where did this energy come from?
How much energy is produced? About 25 MeV (Mega
electron-Volts). This is 1/10,000,000 the amount of energy needed to
lift one drop of water one cm. You use more energy than this just
sitting there. It's not much energy at all. So what does this tell you?
It tells you that there must be an unimaginably large number of
hydrogen atoms in the center of the Sun, all participating in this
reaction all the time, in order to make the Sun as bright as it is!
The Sun consumes 6X1011 kg of hydrogen every second. Once again, we are in a region where it doesn't really matter if you have a good intuition for what the units are, 1011 is a lot of anything.
There is enough hydrogen in the Sun to keep it burning at this
rate for 100 billion years at this rate. But you've probably heard at
some time that the lifetime of the Sun is 10 billion years, and we're
halfway through it... How can this make sense? Well, not all of the Sun
will be fused into helium. Only about 10% of the hydrogen in the Sun
will reach temperatures and pressures high enough to be fused. So the
Sun will live for approximately 10% of 100 billion, or 10 billion
years.
Recall those neutrinos. They could pass through three light
years of lead without even noticing. Do you think they care about a few
light seconds of star stuff? No. They don't. It takes a typical
neutrino only 2 seconds to get out of the Sun, and about 8 minutes to
cross the distance between the Earth and the Sun. Neutrinos are the
best probe of what's happening inside the Sun NOW. But there's a
problem. If neutrinos don't care about ordinary matter, how can we
catch them? They won't interact with photographic film, or digital
cameras, or eyes. So what do we do? Homestake experiment.
600,000 gallons of cleaning fluid buried underground (I suspect I kept saying 600,000 tons. No matter. It's a lot.). If 1016
neutrinos pass through you every second, even more of them pass through
all this cleaning fluid. Once every 12 hours, a neutrino interacts with
a chlorine atom, and turns it into an argon atom. Every 60 days, some
poor slob grad student has to count 'em up.
Only about 1/3 as many neutrinos were found as were predicted.
Two possible solutions to this disagreement between theory and
experiment:
can travel through 3 light years of lead as though it wasn't even there. There are 1016
(10,000,000,000,000,000) neutrinos passing through your body every
second, and you don't even know it. They go in one side and out the
other, and don't even slow down.
A Gamma Ray is really just a high energy bit of light, like an X-ray, only with even more energy.
So you can see that a lot of energy comes out of this reaction.
There's the energy to make the neutrino, and send it flying away, and
the energy in the two gamma rays (one resulting from the
positron-electron annihilation). Where did this energy come from?
The helium atom which we end up with is actually less
massive than the four hydrogen atoms that we began with! All this talk
about mass and energy should make you think immediately (ok, maybe not immediately!) of the most famous formula ever:
E=mc2
In this equation, E stands for energy, m stands for mass, and c is the speed of light, 3X108
m/s. What it says is that mass is just another form of energy, and that
you can turn energy into mass, and mass into energy, according to the
formula. This was one of Einstein's greatest contributions to humanity,
figuring out that mass and energy are really just different forms of
the same thing.
A Gamma Ray is really just a high energy bit of light, like an X-ray, only with even more energy.
So you can see that a lot of energy comes out of this reaction.
There's the energy to make the neutrino, and send it flying away, and
the energy in the two gamma rays (one resulting from the
positron-electron annihilation). Where did this energy come from?
How much energy is produced? About 25 MeV (Mega
electron-Volts). This is 1/10,000,000 the amount of energy needed to
lift one drop of water one cm. You use more energy than this just
sitting there. It's not much energy at all. So what does this tell you?
It tells you that there must be an unimaginably large number of
hydrogen atoms in the center of the Sun, all participating in this
reaction all the time, in order to make the Sun as bright as it is!
The Sun consumes 6X1011 kg of hydrogen every second. Once again, we are in a region where it doesn't really matter if you have a good intuition for what the units are, 1011 is a lot of anything.
There is enough hydrogen in the Sun to keep it burning at this
rate for 100 billion years at this rate. But you've probably heard at
some time that the lifetime of the Sun is 10 billion years, and we're
halfway through it... How can this make sense? Well, not all of the Sun
will be fused into helium. Only about 10% of the hydrogen in the Sun
will reach temperatures and pressures high enough to be fused. So the
Sun will live for approximately 10% of 100 billion, or 10 billion
years.
Recall those neutrinos. They could pass through three light
years of lead without even noticing. Do you think they care about a few
light seconds of star stuff? No. They don't. It takes a typical
neutrino only 2 seconds to get out of the Sun, and about 8 minutes to
cross the distance between the Earth and the Sun. Neutrinos are the
best probe of what's happening inside the Sun NOW. But there's a
problem. If neutrinos don't care about ordinary matter, how can we
catch them? They won't interact with photographic film, or digital
cameras, or eyes. So what do we do? Homestake experiment.
600,000 gallons of cleaning fluid buried underground (I suspect I kept saying 600,000 tons. No matter. It's a lot.). If 1016
neutrinos pass through you every second, even more of them pass through
all this cleaning fluid. Once every 12 hours, a neutrino interacts with
a chlorine atom, and turns it into an argon atom. Every 60 days, some
poor slob grad student has to count 'em up.
Only about 1/3 as many neutrinos were found as were predicted.
Two possible solutions to this disagreement between theory and
experiment:
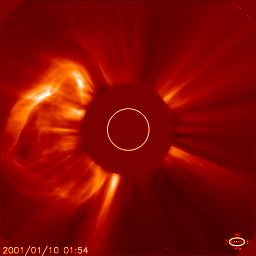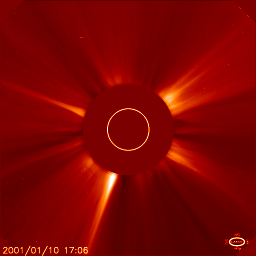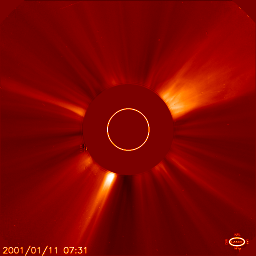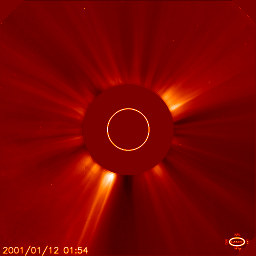 Jan 10: the corona |
 Jan 10: ultraviolet (prominences) |
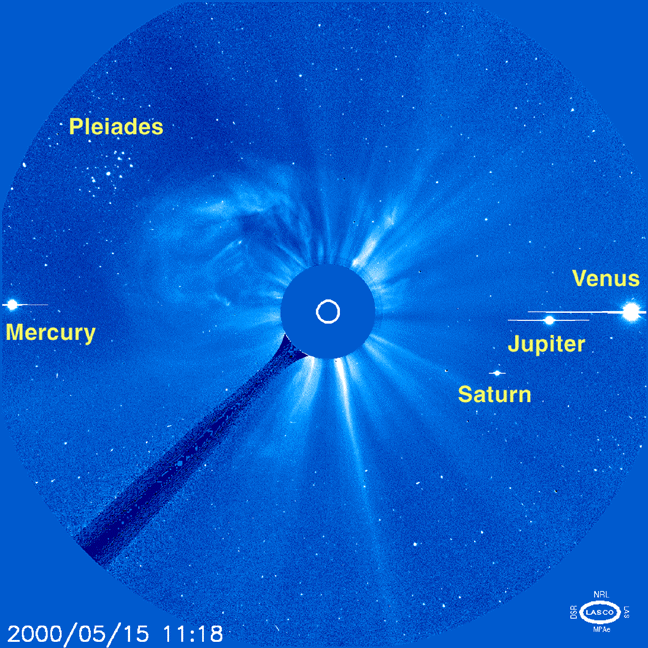 The Sun 'at night' |
 Two comets |
CMEs and p+ showers (Apr, 01) | 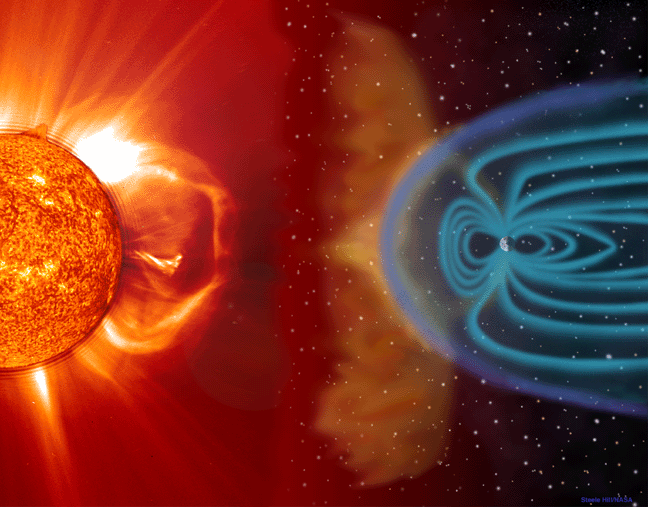 Solar Wind Interaction with Earth |
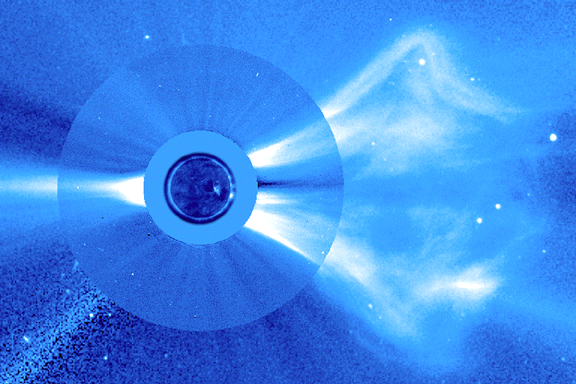 Wide field view of an eruption. |
 Another of the same. |
 Another big eruption. |
An Earth-directed CME | And in the wide-field UV | 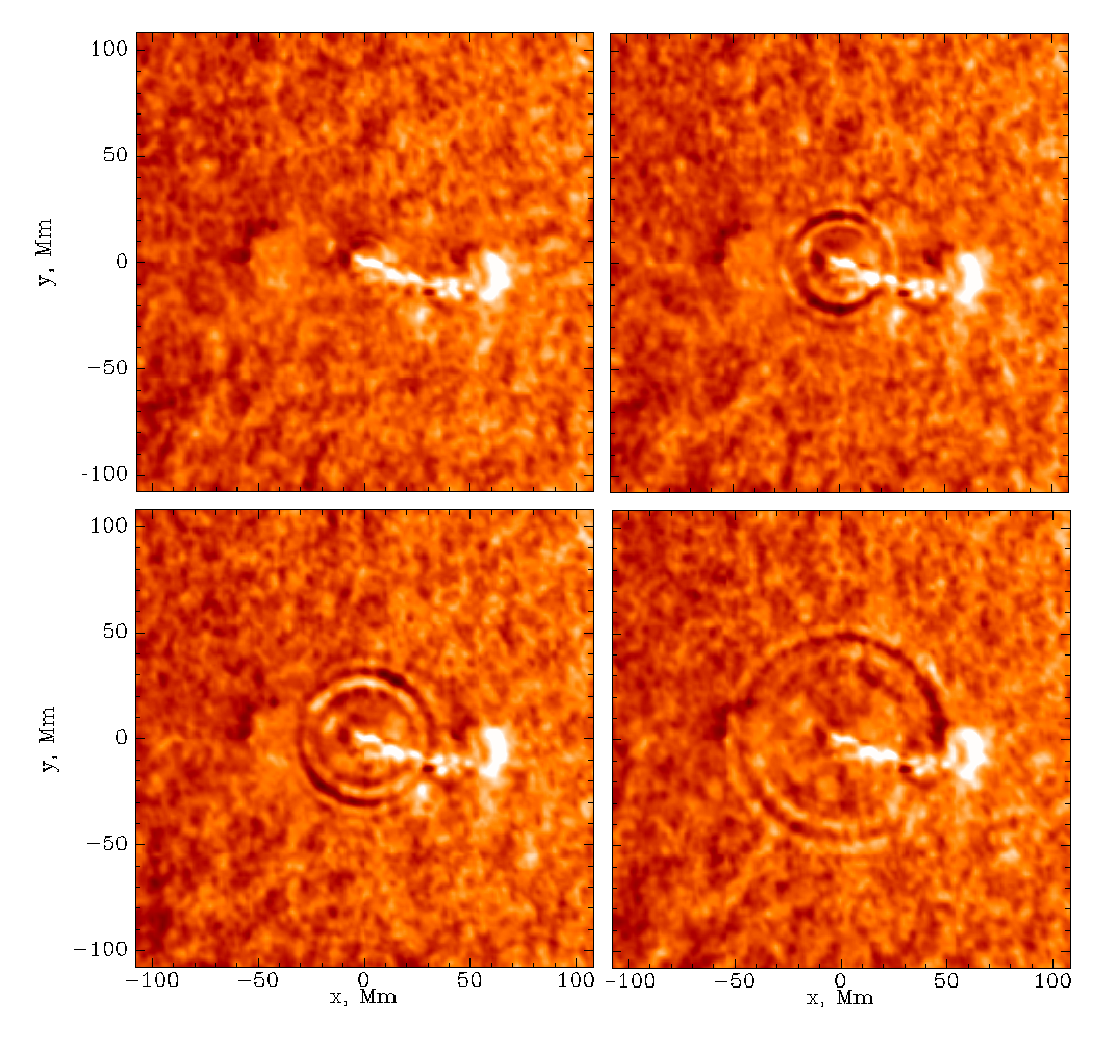 A 'Sun-Quake' |
Aurora movie | |
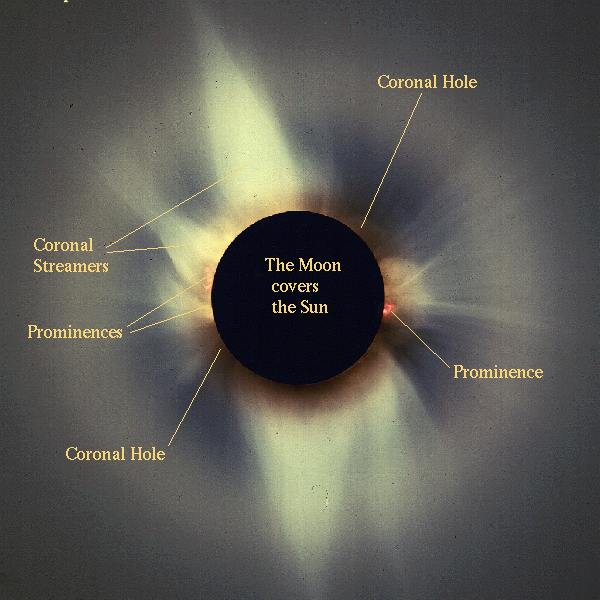 |
eruption movie | prominence sequence | ||||
| Flare with field lines from TRACE | Positron-electron annihilation | SOHO-MDI_views_the_sun, 3 months in 2001 | Spinning sunspot | Sunspot number and UV Flux | ||
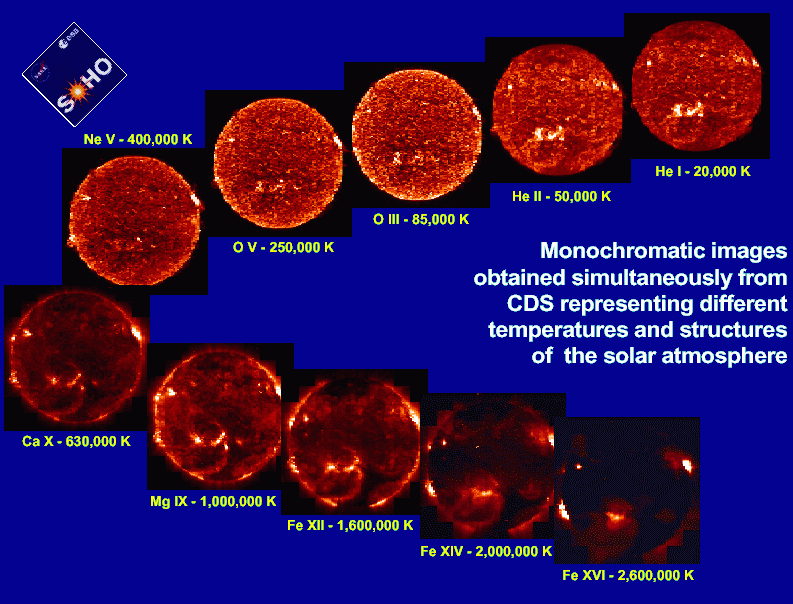 The Sun at different temperatures The Sun at different temperatures |
 A very cool eruptive prominence in 1998 A very cool eruptive prominence in 1998 |
MPEG Movies: Here |