Background and Theory
A spectrum of a star is composed of its continuum emission as well as a number of 'lines' which can be either emission or absorption lines. Emission lines are caused by electrons in atoms or molecules dropping down into lower energy levels and emitting energy, and so appear bright compared to the region of the spectrum around it. Absorption lines cause dark features in the continuum emission where the continuum radiation is removed. This is caused by atoms (or molecules) absorbing radiation, and moving to a higher energy state. This process causes the lines to look darker when compared to the region of the spectrum around them.
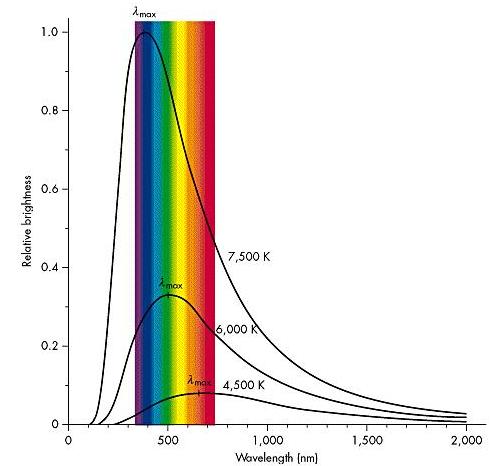
Stars come in a wide range of sizes and temperatures. The hottest stars in the sky have temperatures in excess of 40,000 K, whereas the coolest stars that we can detect optically have temperatures on the order of 2,000-3,000 K. The spectrum of a star is very strongly dependent on its temperature.
Procedure
- The attached panels show spectra of different stellar classes. How are they all the same? How do they differ?
- The lines at about 4340 and 4860 angstroms are critical lines for classifying stars. These are two of the "Balmer lines" of hydrogen, in which the atom is making a transition up from the second energy level to a higher one. Circle the Balmer lines in each panel.
- Put the panels in order of strongest (deepest and widest) to weakest (shallowest and narrowest) Balmer lines. Assign the strongest panel the letter A, next strongest B, next F, G, K, M, and O.
- Continuum emission is the overall trend of the spectrum. In panel six, for example, the spectrum rises at the left of the graph, peaks between 4000 and 5000 Angstroms, and slowly declines again. What is the peak wavelength (
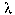 max) of the continuum emission in each panel?
max) of the continuum emission in each panel?
- Blue light has short wavelengths, and red light has longer wavelengths. Which panel has the hottest stars? Which panel has the coolest stars? Put the stars in order from hottest to coolest.
- Is your list in order of line strength the same as the list in order of temperature? Rearrange the letters you assigned for line strength so that they now go in order of temperature.
- But why do O stars and K stars BOTH have weak lines? Hydrogen has only one electron, which is usually in the ground state. But as the temperature rises, the average electron gains more energy from collisions with other atoms, moving up to the second energy level, then the third, and so on. If the hydrogen is hot enough, the electrons leave the atom entirely, so that it becomes ionized. There is a particular temperature at which the average electron will be in the second energy level. At this temperature, LOTS of atoms can absorb Balmer line photons, so stars of this temperature will have the strongest Balmer lines. Which spectral class corresponds to this temperature?
- To find out what the actual temperature is, we need one more step. Using the peak wavelength (
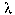 max) of the star (from step 4), and the following formula, find the temperature of the star with the strongest Balmer lines.
T=2.9x107/
max) of the star (from step 4), and the following formula, find the temperature of the star with the strongest Balmer lines.
T=2.9x107/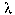 max.
where
max.
where 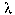 max is in angstroms, and T is in Kelvin. What is the temperature at which the Balmer lines are the strongest? What happens to the Balmer lines at higher temperature? What happens to the Balmer lines at lower temperature?
max is in angstroms, and T is in Kelvin. What is the temperature at which the Balmer lines are the strongest? What happens to the Balmer lines at higher temperature? What happens to the Balmer lines at lower temperature?














