 |
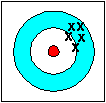 |
 |
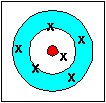
| accurate (the average is accurate) not precise |
precise not accurate |
accurate and precise |
There are two kinds of numbers in the world:
Accuracy refers to how closely a measured value agrees with the correct value.
Precision refers to how closely individual measurements agree with each other.
 |
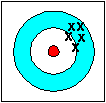 |
 |
| accurate (the average is accurate) not precise |
precise not accurate |
accurate and precise |
A rule of thumb: read a measurement to 1/10 or 0.1 of the smallest division. This means that the error in reading (called the reading error) is ![]() 1/10 or 0.1 of the smallest division on the ruler or other instrument. If you are less sure of yourself, you can read to 1/5 or 0.2 of the smallest division.
1/10 or 0.1 of the smallest division on the ruler or other instrument. If you are less sure of yourself, you can read to 1/5 or 0.2 of the smallest division.
Rules for Significant Figures:
EXAMPLES:
| Example |
Number of Significant Figures | Scientific Notation | ||
| 0.00682 | 3 | 6.82 x 10 -3 | Leading zeros are not significant. | |
| 1.072 | 4 | 1.072 (x 10 0) | Imbedded zeros are always significant. | |
| 300 | 1 | 3 x 102 | Trailing zeroes are significant only if the decimal point is specified. | |
| 300. | 3 | 3.00 x 102 | ||
| 300.0 | 4 | 3.000 x 102 |
EXAMPLES
| Addition |
 | Even though your calculator gives you the answer 8.0372, you must round off to 8.04. Your answer must only contain 1 doubtful number. Note that the doubtful digits are underlined. |
| Subtraction |
 | Subtraction is interesting when concerned with significant figures. Even though both numbers involved in the subtraction have 5 significant figures, the answer only has 3 significant figures when rounded correctly. Remember, the answer must only have 1 doubtful digit. |
| Multiplication |  | The answer must be rounded off to 2 significant figures, since 1.6 only has 2 significant figures. |
| Division |  | The answer must be rounded off to 3 significant figures, since 45.2 has only 3 significant figures. |
Notes on Rounding
| Question 1 | Give the correct number of significant figures for 4500, 4500., 0.0032, 0.04050 |
| Question 2 | Give the answer to the correct number of significant figures: 4503 + 34.90 + 550 = ? |
| Question 3 | Give the answer to the correct number of significant figures: 1.367 - 1.34 = ? |
| Question 4 | Give the answer to the correct number of significant figures: (1.3 x 103)(5.724 x 104) = ? |
| Question 5 | Give the answer to the correct number of significant figures: (6305)/(0.010) = ? |
