Kirchoff's Laws
Purpose:
This exercise is collaborative, and helps the students to understand Kirchoff's laws in a more kinesthetic way. The students work together to make a model of a gas cloud and incident photons. The reason for the difference between emission and absorption lines quickly becomes clear.
Audience:
This activity has the potential to become complete chaos in immature audiences. It works best with college students, and groups of mature young adults.
Materials: (for each 7-10 students)
- 70 tennis balls, ten each red, orange, yellow, green, blue, indigo, violet
- 9 small buckets
- 2 large buckets
- colored pencils
Activity:
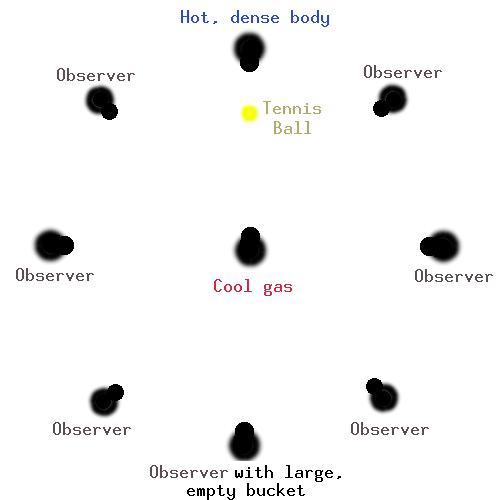
- The group of 10 students form a circle around a student in the center, about 5 m across.
- One student is the hot dense object, and takes a large bucket with ALL the balls in it and a small bucket.
- The center student is the gas cloud.
- The other students are given a small bucket, except the student directly opposite the 'hot, dense object', who takes the large (empty) bucket.
- THIS IS AN IMPORTANT STEP. DO NOT SKIP IT! Explain what the students will do, and ask them to PREDICT what will happen. Write these predictions on the board, so that you can refer to them later.
- The hot dense object tosses the balls to the person in the center, (one ball at a time!) The center student catches only the orange and blue balls, and throws them to a random student (including the student behind them, and the hot dense object).
- The student with the large bucket catches all the balls that come her way.
- When a student catches a ball, they add it to their bucket.
- After all the balls have been thrown (and caught!), the students each make a spectrum on the board representing the number of balls of each color. Have the spectra drawn in locations representing where the students were in the circle.
- If there are too many students, the others can either watch, and make sure everyone's doing it right, or form a separate circle (if there are enough tennis balls).
Discussion:
- Compare the results of the 'experiment' with the predictions.
- Ask the students to predict what someone would observe if they were nearly, but not exactly opposite the hot, dense object.
- Ask the students to predict what an observer would see if they were BETWEEN the hot dense body and the gas cloud, looking towards the hot dense object, and looking towards the gas cloud.
- Ask the students how they might expect solar lines to look if viewed in the sky a) towards the Sun, and b) away from the Sun. Try it with diffraction gratings and Mylar sheets. (Remind the students to not look directly at the Sun!)
For Homework:
- Write up the results, comparing the predictions with the results, and explaining what misconceptions were present in the predictions (i.e. why they predicted the wrong thing, or the right thing for the wrong reasons.)
- Have the students suggest ways to improve the accuracy of this representation. For example, having the 'gas cloud' step up onto books of different heights depending on which ball they've caught, and then step down when they throw the ball would more accurately represent the changing energy state of the cloud.
