Any object with a temperature above absolute zero emits light at all wavelengths. If the object is perfectly black (so it doesn't reflect any light), then the light that comes from it is called blackbody radiation.
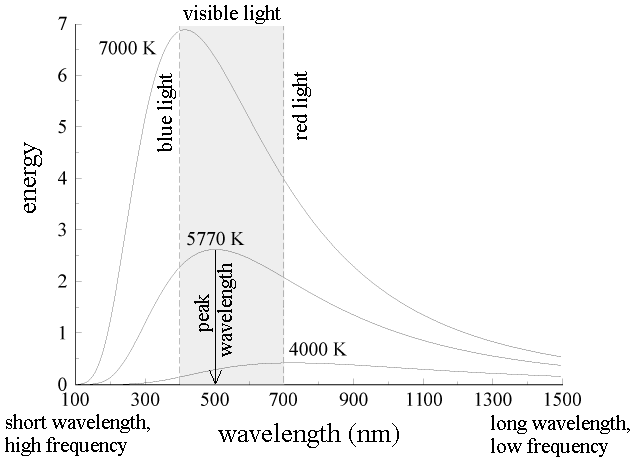
The energy of blackbody radiation is not shared evenly by all wavelengths of light. The spectrum of blackbody radiation (below) shows that some wavelengths get more energy than others. Three spectra are shown, for three different temperatures. (One of the curves is for the surface temperature of the Sun, 5770 K.)

Here are some experimental facts about blackbody radiation:
a. The blackbody spectrum depends only on the temperature of the object, and not on what it is made of. An iron horseshoe, a ceramic vase, and a piece of charcoal --- all emit the same blackbody spectrum if their temperatures are the same.
b. As the temperature of an object increases, it emits more blackbody energy at all wavelengths.
c. As the temperature of an object increases, the peak wavelength of the blackbody spectrum becomes shorter (bluer). For example, blue stars are hotter than red stars.
d. The blackbody spectrum always becomes small at the left-hand side (the short wavelength, high frequency side).
The explanation of classical physics: Light is an electromagnetic wave that is produced when an electric charge vibrates. (Strictly speaking, "vibrates" means any change in how the charge moves --- speeding up, slowing down, or changing direction.) Now recall that heat is just the kinetic energy of random motion. In a hot object, electrons vibrate in random directions and produce light as a result. A hotter object means more energetic vibrations and so more light is emitted by a hotter object --- it glows brighter. So far, so good. But classical physics could not explain the shape of the blackbody spectrum.
The electrons in a hot object can vibrate with a range of frequencies, ranging from very few vibrations per second to a huge number of vibrations per second. In fact, there is no limit to how great the frequency can be. Classical physics said that each frequency of vibration should have the same energy. Since there is no limit to how great the frequency can be, there is no limit to the energy of the vibrating electrons at high frequencies. This means that, according to classical physics, there should be no limit to the energy of the light produced by the electrons vibrating at high frequencies. WRONG!! Experimentally, the blackbody spectrum always becomes small at the left-hand side (short wavelength, high frequency).
At about 1900, Max Planck came up with the solution. He proposed that the classical idea that each frequency of vibration should have the same energy must be wrong. Instead, he said that energy is not shared equally by electrons that vibrate with different frequencies. Planck said that energy comes in clumps. He called a clump of energy a quantum. The size of a clump of energy --- a quantum --- depends on the frequency of vibration. Here is Planck's rule for the a quantum of energy for a vibrating electron:
energy of a quantum = (a calibration constant) x (frequency of vibration)
or
E = hf
where h, the calibration constant, is today called Planck's constant. Its value is about 6 x 10-34, very tiny!
So how does this explain the spectrum of blackbody radiation? Planck said that an electron vibrating with a frequency f could only have an energy of 1 hf, 2 hf, 3 hf, 4 hf, ... ; that is,
energy of vibrating electron = (any integer) x hf
But the electron has to have at least one quantum of energy if it is going to vibrate. If it doesn't have at least an energy of 1hf, it will not vibrate at all and can't produce any light. "A ha!" said Planck: at high frequencies the amount of energy in a quantum, hf, is so large that the high-frequency vibrations can never get going! This is why the blackbody spectrum always becomes small at the left-hand (high frequency) side.
When light shines on the surface of a metallic substance, electrons in the metal absorb the energy of the light and they can escape from the metal's surface. This is called the photoelectric effect, and it is used to produce the electric current that runs many solar-powered devices. Using the idea that light is a wave with the energy distributed evenly throughout the wave, classical physicists expected that when using very dim light, it would take some time for enough light energy to build up to eject an electron from a metallic surface. WRONG!! Experiments show that if light of a certain frequency can eject electrons from a metal, it makes no difference how dim the light is. There is never a time delay.
In 1905, Albert Einstein came up with the solution. If Max Planck's idea that energy comes in clumps (quanta) is correct, then light must consist of a stream of clumps of energy. Each clump of light energy is called a photon, said Einstein, and each photon has an energy equal to hf (Planck's constant times the frequency of the light). Therefore the energy of light is not evenly distributed along the wave, but is concentrated in the photons. A dimmer light means fewer photons, but simply turning down the light (without changing its frequency) does not alter the energy of an individual photon. So for a specific frequency light, if a single photon has enough energy to eject an electron from a metallic surface, then electrons will always be ejected immediately after the light is turned on and the photons hit the metal.
When a small tube of hydrogen gas is heated, it begins to glow and emit light. Unlike the blackbody radiation that comes from a hot dense solid or gas, this light consists of just a few colors (wavelengths): a red wavelength, a turquoise, and several violets. Classical physicists at the beginning of the century thought they should certainly be able to understand hydrogen, since it is the simplest atom. Hydrogen consists of a positively charged proton at the center, with a negatively charged electron orbiting around it. The electrical attraction between the positive proton and the negative electron keeps the electron in orbit, just like the gravitational attraction between the Sun and the Earth holds the Earth in orbit. There was just one problem. Classical physics said that because the orbiting electron is constantly changing direction, it should emit electromagnetic radiation --- light. As a result, the electron should be continually losing energy. In fact, physicists calculated that the electron should lose all of its energy and spiral down into the proton in only about 0.000000000001 second! In other words, atoms should not exist longer than a mere 10-12 seconds. WRONG!!
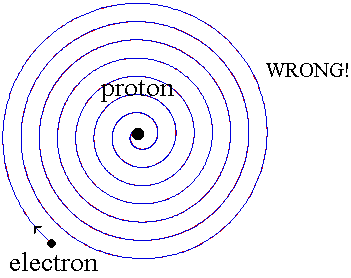
Niels Bohr provided an explanation in 1913. In the Bohr model of the hydrogen atom, the electron can't orbit the proton in any size orbit it pleases. There are only certain allowed orbits, and each allowed orbit has a certain radius and a certain energy. Bohr invented a rule that allowed him to calculate the size and energy of each orbit. If you are curious, Bohr's rule said that
2π x (electron mass) x (electron orbital speed) x (orbit radius) = (any integer) x h
which is not too obvious, to say the least! (The integer would be 1 for the smallest orbit, 2 for the next orbit out, and so on.) Bohr also made up a new rule to explain the stability of the hydrogen atom --- why it could last longer than 0.000000000001 second. He said that when an electron is in an allowed orbit, the electron will not produce electromagnetic radiation. Bohr did not explain why, he just proposed a new law of nature. And nature agreed with Niels Bohr. His new model of hydrogen gave wavelengths for hydrogen gas that precisely agreed with what was measured.
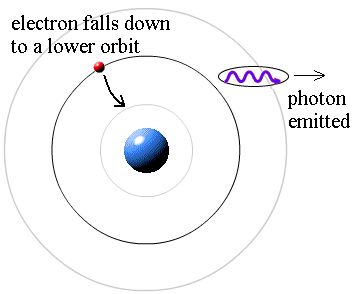
Question: If the electrons do not produce light when they are in their allowed stable orbits, where is the source of the light that comes from hydrogen? Answer: According to Bohr, electrons have more energy when they are in larger orbits. If an electron falls from a larger orbit down to a smaller orbit, it loses energy. According to the law of conservation of energy, the energy lost by the electron must go somewhere. Bohr explained that a photon carries away the lost energy from the hydrogen atom; that is,
photon energy = (electron energy in larger orbit) - (electron energy in smaller orbit)
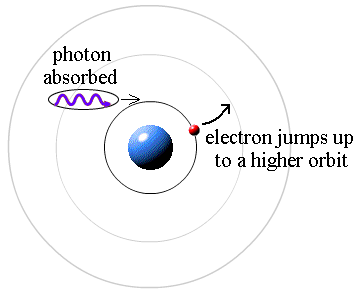
It works the other way, too. If a photon strikes an atom, the atom can absorb the photon and its energy if (and only if) the photon's energy is exactly equal to the difference between two orbital energies. In this case, an electron uses the photon's energy to jump from the smaller orbit up to the larger orbit. This is called a quantum jump.
 |
 |
|
|
When light passes through a double-slit, an interference pattern consisting of bright bands and dark bands is seen on a screen. This is produced when the wave from one slit combines with the wave from the other slit. If two wave crests meet at the screen, the waves add and you get a bright band. If a wave crest from one slit meets a wave trough from the other slit, the waves cancel and you get a dark band. This proves that light is a wave.
On the other hand, the photoelectric effect proves that light consists of massless particles called photons.
So which is it? Is light a wave or a stream of particles? The answer is "Yes!"
Light acts like a wave if you want to know how it propagates, how it travels from one place to another. To describe how light travels from the double slits to the screen, you have to use the wave characteristics of light.
Light acts like particles (photons) if you want to know how light interacts with matter. To describer how light interacts with the electrons in a metal and how it ejects them from the metal's surface, you have to use the particle characteristics of light.
We say that light exhibits a wave-particle duality. It can behave like either waves or particles (but not both at the same time), depending on the situation.
Thinking about the photoelectric effect again, how can a photon (which has no mass) knock an electron about? Einstein used his theory of relativity to show that even massless photons have momentum. Newton defined momentum = (mass) x (velocity) for a particle with mass, but Einstein was able to show that the momentum of a massless photon depends on its wavelength:
The smaller the wavelength, the greater the momentum of the photon.
In 1923, Prince Louis de Broglie of France had an idea. Maybe the wave-particle duality applies to everything in nature. He proposed that everything propagates like a wave, and that everything interacts like a particle. Say what?? What do you mean by the wavelength of an electron, or the wavelength of a baseball? De Broglie rewrote Einstein's formula for the momentum of a photon and applied it to a particle with mass:
Planck's constant, h, is so tiny that we don't notice the wavelength of a thrown baseball, which is only about 10-35 meters! But an electron's mass is also tiny, so it has a wavelength about 10,000 times shorter than the wavelength of visible light. This is useful, because microscopes that use electron waves instead of light waves can see several thousand times more detail!
The proof that electrons propagate like a wave came when electrons were passed through a double slit and counted as they hit a screen. If the electrons traveled like a stream of particles, they would have simply piled up at two locations behind the two slits. But they didn't. They showed a double-slit interference pattern, bright bands and dark bands just like the ones produced by light waves. Without a doubt, electrons exhibit the wave-particle duality of nature. In fact, every massive object exhibits the wave-particle duality of nature. It just isn't noticeable on the large scale of our everyday world.
If you look at most of the "equations" above, you will find Planck's constant, h. This is the trademark of "modern physics." The failure of classical physics to explain blackbody radiation, the photoelectric effect, and the hydrogen atom ultimately demolished the foundations of classical physics.
Max Planck, Albert Einstein, Niels Bohr, and Louis de Broglie made inspired guesses about how nature works. Other people of their time made different guesses. Nature agreed with Planck, Einstein, Bohr, and de Broglie, but not with the others whose names are now forgotten. Like Arcadia's Thomasina, these were intuitive geniuses who went beyond mere mathematics to make creative conjectures about how the world operates. Those who came later calculated the consequences of the new physics, but this was just mathematics (sometimes brilliant mathematics, but mathematics nonetheless). It is those rare intuitive geniuses who courageously discard the old rules and invent new ones who are in the first rank of physicists.
Finally, It is important to remember that Planck's constant is very tiny, only about 6 x 10-34. Roughly speaking, this means that in our everyday world, quantum effects like the wave-particle duality make a difference only in the 34th decimal place when predicting the behavior of a moving baseball. Large objects obey Newton's laws. But the behavior of large object reflect the average behavior of their component atoms, so it is fair to say that Newton's laws work only "on average." The behavior of small systems is radically different than what classical physics predicts. Ultimately, the whole idea of prediction --- that the same conditions should always produce the same results --- was overthrown. This is explained in Chapter 6 of Richard Feynman's The Character of Physical Law.
Return to Honors 1500 home page
Last modified: Sunday, May 08, 2005 06:03 PM