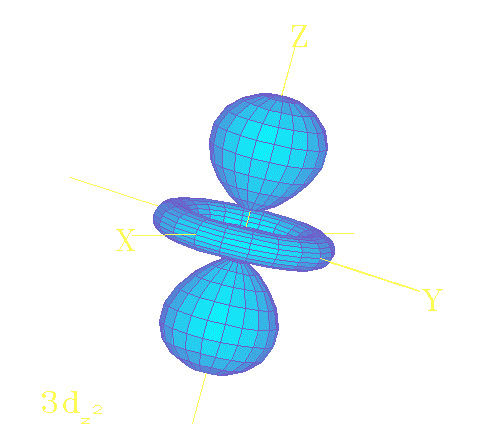The solutions
of the Schrodinger equation
describe the electron wave patterns
that give atoms their properties!
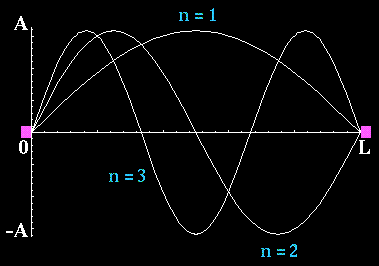
Waves on a vibrating string
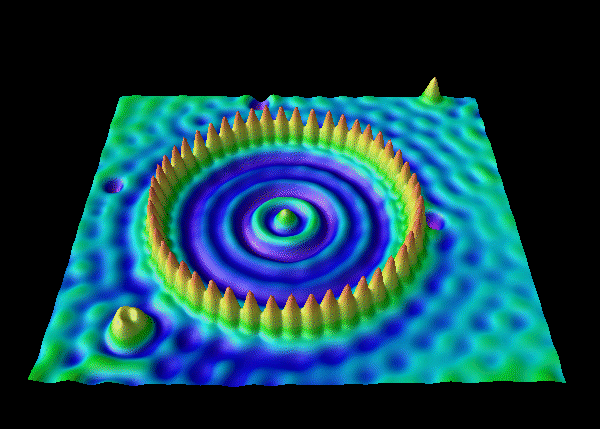
electron waves of an electron trapped in a corral of iron atoms
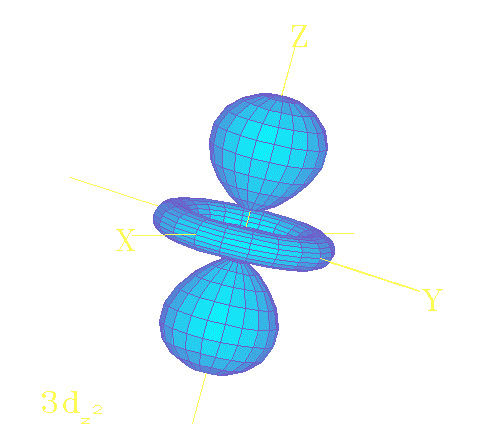
An electron wave pattern (orbital) of hydrogen
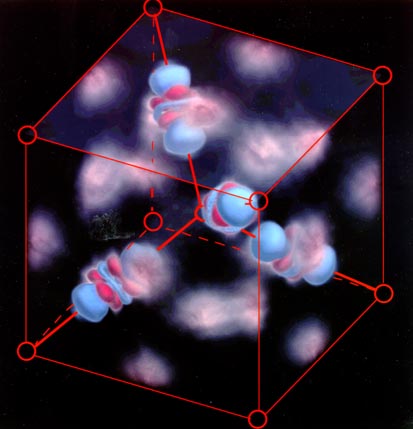
Images -- not computer simulations -- of dumbbell-shaped clouds of
electrons shared between copper and oxygen atoms in cuprite (Cu2O). The nuclei
of the copper atoms (not shown) are at the center of the blue and red shaded orbitals.
Superimposed red circles represent the locations of oxygen nuclei. (Photo
courtesy Jian-Min Zuo, Miyoung Kim, Michael O'Keefe and John Spence, Arizona State
University.)
If any of the three numbers in the Schrodinger
equation ---
Planck's constant: h (determines size of
electron waves)
the electron's mass: me
the electron's charge: e
--- were even slightly different,
our physical world would by profoundly changed!
index | back
| next


 symmetric
vibration of a drumhead (1.44 Mb movie)
symmetric
vibration of a drumhead (1.44 Mb movie) asymmetric vibration
of a drumhead (1.44 Mb movie)
asymmetric vibration
of a drumhead (1.44 Mb movie)